

Grifols North Fractionation Biotech Facility - EPC
Client: Grifols
Location: Clayton, NC, U.S.
Business Segment: Urban Solutions
Industries: Life SciencesTechnology

Executive Summary
Grifols, a global biotherapeutic and biotechnology company, selected Fluor to significantly expand their manufacturing capacity. The scope included engineering, procurement, construction management and commissioning of the North Fractionation Facility, or NFF project. Augmenting the existing Clayton facility in North Carolina, the NFF project included a 160,000-square-foot fractionation expansion, allowing Grifols a 43% increase over their previous plasma processing capacity.
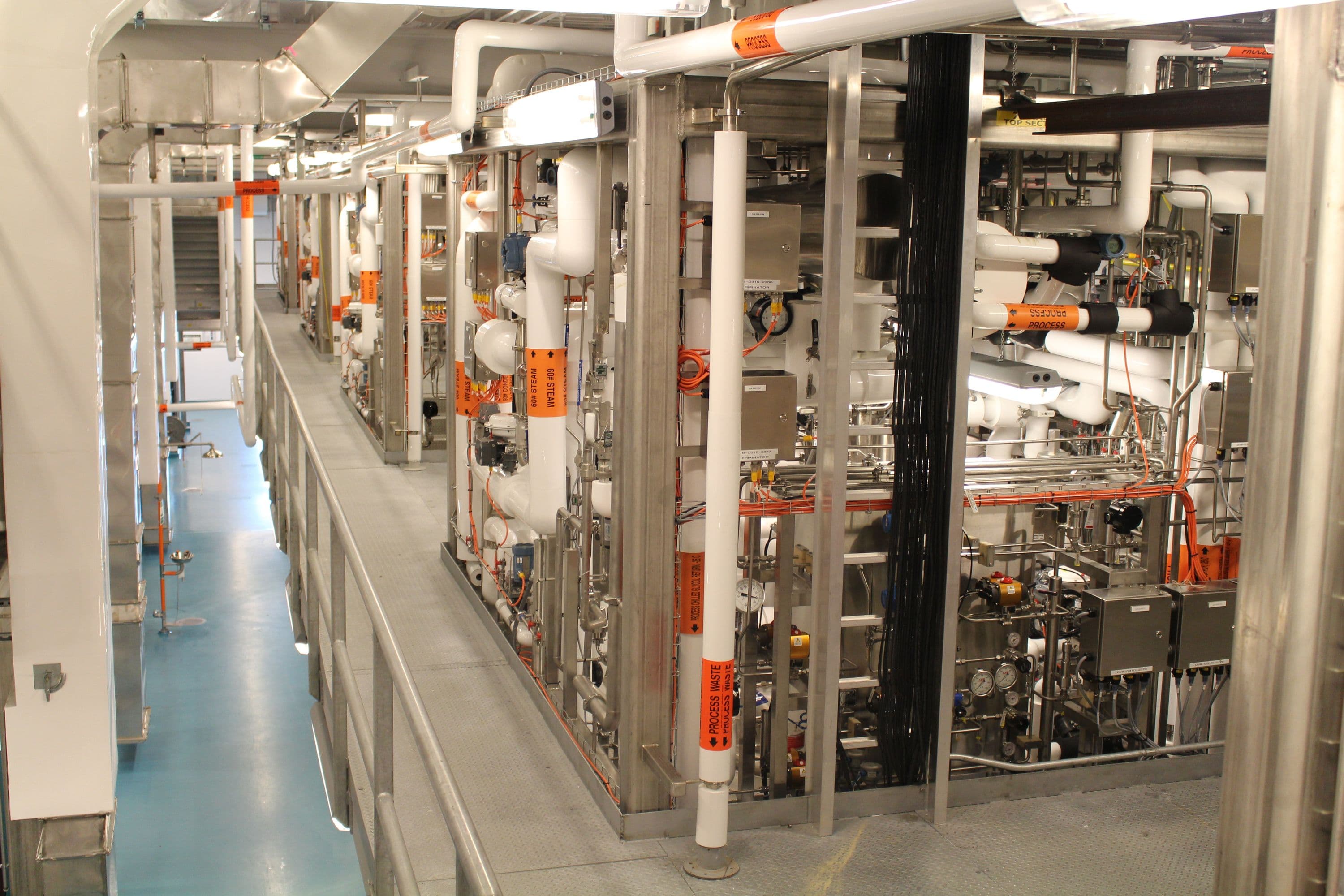
The NFF project included new centrifuge technology, expansion of existing plant utilities and systems and revamp of an existing ethanol fractionation facility. Fluor provided an integrated approach utilizing modular construction expertise that facilitated an aggressive schedule date.
In 2014, Grifols' North Fractionation Facility was named the International Society for Pharmaceutical Engineering (ISPE) 2014 Facility of the Year for Project Execution.
Client's Challenge
Grifols is a leader in the research, development and production of critical care treatments for people with rare, chronic and often life-threatening conditions. Grifols products are used for very rare diseases, specifically antitrypsin and different immune deficiencies.
The new fractionation facility expansion receives blood plasma as the raw material, fractionates it and manufactures products for blood therapies. The target population of patients who have blood diseases is typically very small, but the need for reliable supply is critical since patients rely heavily on their products. Previously, the Grifols operation in Clayton had a fractionation capacity of 4 million liters. The new facility provides the opportunity to fractionate up to 6 million additional liters of blood plasma.
The 160,000-square-foot NFF project included new centrifuge technology, expansion of existing plant utilities and systems and revamp of an existing ethanol fractionation facility. Similar to biotechnology operations, high-grade stainless steel is required for the process equipment and the clean utilities; e.g., water for injection, reverse osmosis water. All the process transfer units were built in 316 low-carbon stainless steel and polished internally to achieve a 20 Ra surface finish.

Fluor's Solution
The team started conceptual design and preliminary engineering in 2009. In spring 2010, the Clayton site was cleared and steel erection commenced in early summer. Construction was completed in 2012, and then the commissioning and qualification phase was entered. In addition to onsite Fluor resources in Clayton, we leveraged life sciences experts based at our Greenville, South Carolina office.
The Grifols project employed advanced modular design and construction techniques to build both the NFF structure and processing units simultaneously in order to shorten the overall schedule. Rather than a sequential construction approach, where the building is first constructed, then process units are installed, piped and finally started up, the NFF construction strategy was based upon a concurrent build model.
As an example, the Precipitation Super Skids were designed ahead of the building itself. These units, which were too large to be transported over the road, were assembled on site. The completed assemblies were then transferred and installed into the building, completely tested and ready for commissioning and start-up. Joint coordination of the design and modular construction of these high-grade stainless steel processing modules was an activity critical to the project's success.

Conclusion
For decades, Fluor has delivered complex projects for pharmaceutical and biotechnology clients around the world. We brought this approach to the Grifols NFF Project in Clayton, North Carolina. The NFF provides additional manufacturing capacity to produce critical-care blood therapy products. Grifols and Fluor utilized modular design/construction techniques to build the NFF and processing units simultaneously to facilitate an aggressive schedule.
In 2014, Grifols' North Fractionation Facility was named the International Society for Pharmaceutical Engineering (ISPE) 2014 Facility of the Year for Project Execution.

Project Gallery





&w=3840&q=75)
&w=3840&q=75)

&w=3840&q=75)