
&w=3840&q=75)
Genentech Growth Hormone Development Manufacturing Facility
Client: Genentech
Location: San Francisco, CA, U.S.
Business Segment: Urban Solutions
Industry: Life Sciences

Executive Summary
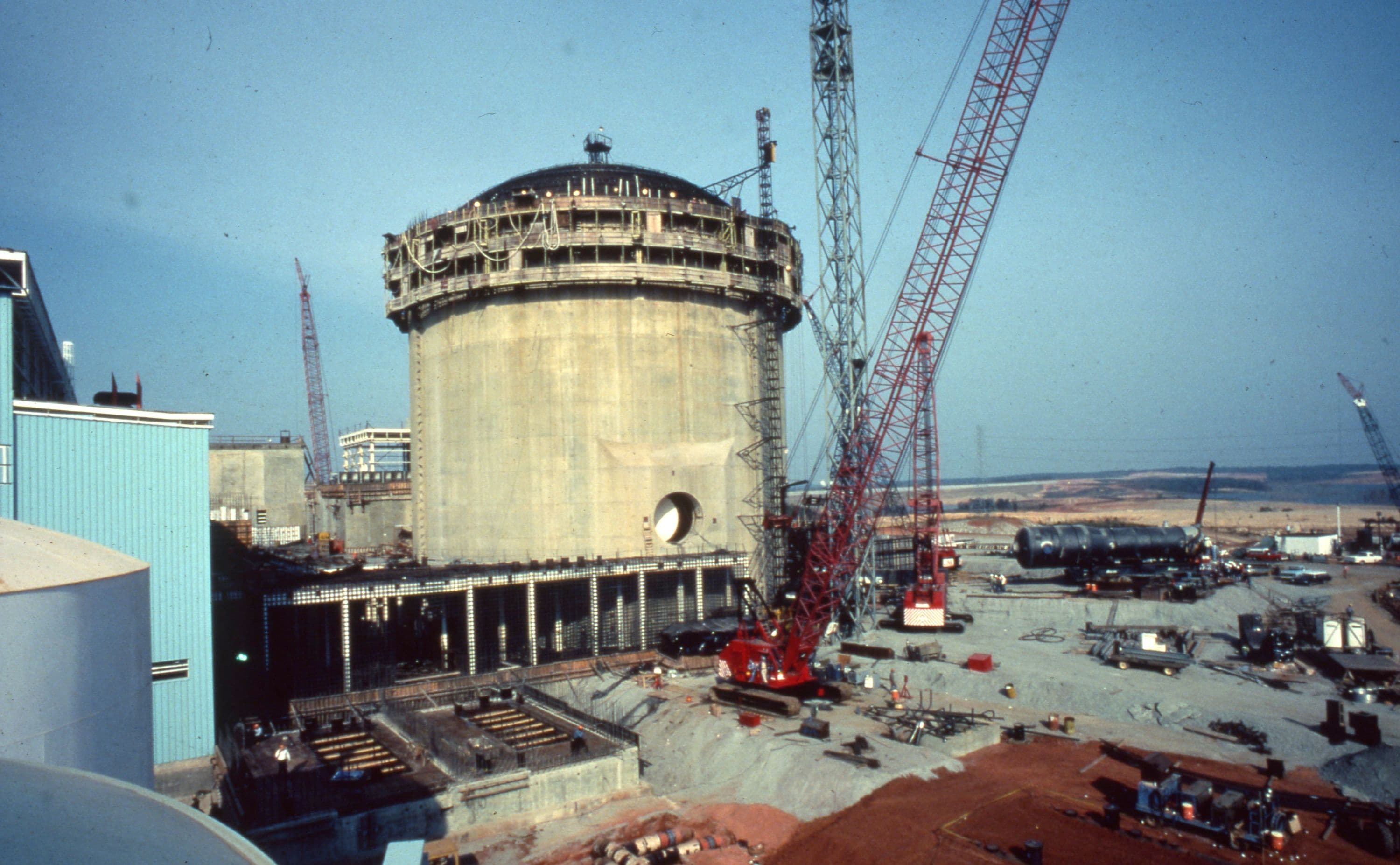
Genentech, the first independent genetic research company to build its own manufacturing facility, selected Fluor to provide complete engineering design, procurement and construction management services for a new 72,000-square-foot building. At the time, it was the largest multi-product genetic engineering and manufacturing plant in the world.
Client's Challenge
The facility produced a variety of products, such as human growth hormone and interferon, made by fermentation processes involving genetically modified microorganisms.
The project included laboratories, semi works and production-scale fermentation processing controlled by advanced computer systems. Separation and purification processes designed for growth hormone and other proteins involved the first commercial scale-up of protein separation technology. The facility's extensive, large-scale modular laboratories, cold rooms and development modules were designed to be interchanged on short notice as Genentech's requirements dictated.

Fluor's Solution
Provisions for expansion allowed for substantial growth within the confines of the existing facility. Designed within the guidelines established by the National Institute of Health for work with recombinant DNA-modified organisms, the plant's production operations also complied fully with the U.S. Food and Drug Administration's Current Good Manufacturing Practices.
Using bench-scale data, Fluor process engineers worked closely with Genentech's research personnel to establish process definition, process flow diagrams, piping and instrumentation diagrams and equipment data sheets for production scale-up.

Conclusion
Fluor completed the $25 million project in only 16 months from conceptual design to start-up. Construction was carried out using a construction management merit-shop subcontracting approach.

&w=3840&q=75)
